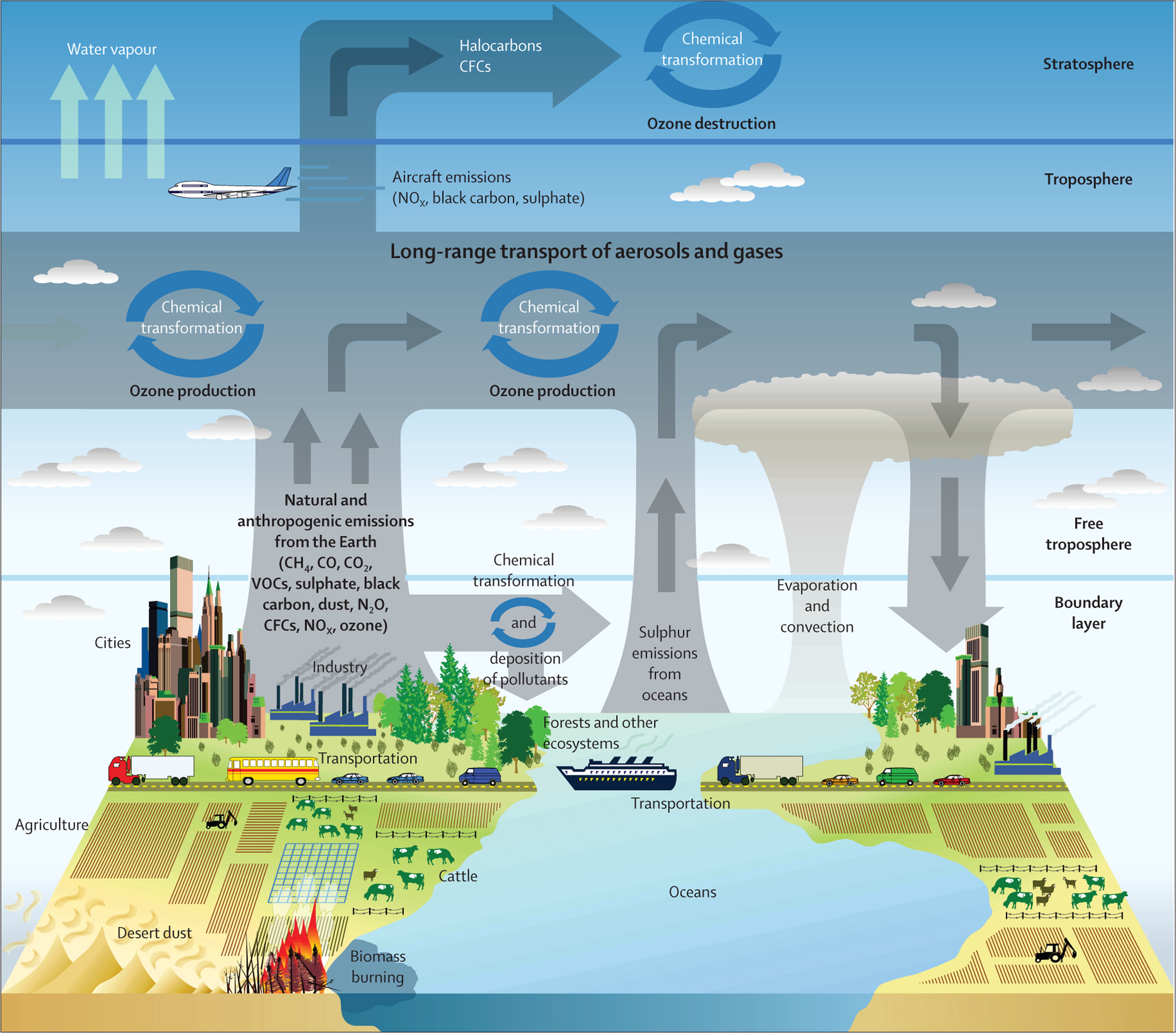
1.5. Interactions between (geo)physical and chemical processes in the atmosphere
Meteorological conditions affect atmospheric chemical processes and, in a few cases, the reverse is also true. The following list contains a selection of these interactions; readers are invited to further investigate each of these topics themselves.
- Wind speed and direction: with more wind, more mixing and dispersion takes place, as well as more (long-range) transport of dust and pollutants. A very low wind speed keeps pollutants near their sources, which can lead to high concentrations. A highly variable wind direction causes the pollutants to be dispersed in all directions. Finally, low wind speed can facilitate gas and particle deposition while high wind speed can cause resuspension of previously deposited particles.
- When (large scale) synoptic systems bring a 'cleaner' air mass, concentrations of pollutants can reduce, whereas if they bring a polluted air mass, local pollution levels increase.
- Atmospheric chemistry (formation and degradation of pollutants) is affected by the air temperature, humidity, and solar radiation (including the effect of clouds). For example, low temperature and high humidity conditions favour the conversion of gaseous ammonia to particulate ammonium (a secondary inorganic aerosol).
- Air temperature inversions in the lowest layer of the atmosphere and high-pressure inversions can cause accumulation of pollutants in the lowest layer of the atmosphere. As a given amount of pollutant is trapped in a smaller volume, under higher pressure, the resulting pollutant concentration is higher.
- Precipitation washes out pollutants: raindrops and snowflakes absorb gases and particles. Raindrops are more efficient at absorbing water soluble and/or small molecules, whereas snow is more effective at absorbing non-water soluble and larger molecules (Lei and Wania, 2004).
- Saharan dust intrusions and volcanic ash can reduce the surface layer air temperature, due to less solar radiation reaching the surface (dust particles reflect the sunlight).
- Clouds can occur unexpectedly: certain atmospheric conditions and aerosol (dust or ash) intrusion can cause cloud formation.
- Less mixing occurs in valleys, and air pollution from within valleys can accumulate at the bottom. In cities, 'artificial canyons' show similar effects.
- Smog (smoke + fog) formation occurs when fog is present at the same time as air pollution. The fog then becomes heavier and darker due to the smoke and chemical fumes. Occurrences of smog in Europe are quite rare these days.
Question 3
Where do you expect the highest PM10, and nitrogen dioxide concentrations? See Figure 1.1. Click on the image to enlarge it.
Question 4
Which of the following is true?
Question 5
How many of the European limit values are below the WHO guidelines?
Question 6
Imagine it is winter and there is a surface inversion. You work in the weather room and receive phone calls from people who complain about having headaches and itchy throats. They ask you if any of these symptoms could be due to the air quality and what you would recommend.
Question 7
Which of the following could be related to a Sahara dust event? Select all that apply.
Question 8
Which atmospheric conditions can favour an air pollution event? You can choose multiple answers.