1.3. Nitrogen dioxide as an air pollution indicator
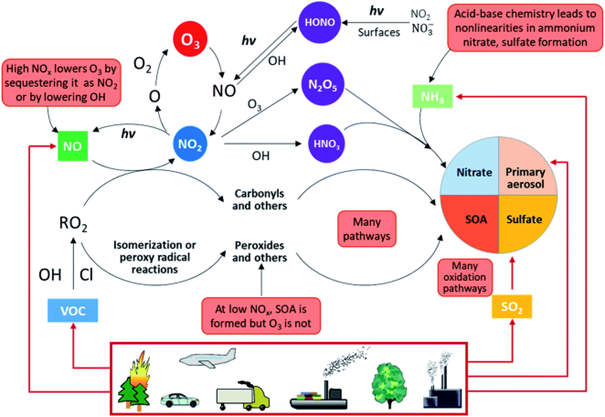
Nitrogen dioxide can be considered an indicator of air quality in urban and industrialized areas. In urban environments (Figure 1.3), nitrogen dioxide is primarily emitted from fossil fuel combustion as nitrogen oxide (NO). This nitrogen oxide plays an important role in atmospheric chemistry by rapidly reacting with ozone or radicals and forming nitrogen dioxide. In the presence of solar light (hv in Figure 1.3), nitrogen dioxide is photolyzed by short-wave solar radiation to nitrogen monoxide and free oxygen. At a local scale, this equilibrium of the NO−NO2−O3 system in the lower troposphere is dominated by a limited number of fast reactions (including carbon monoxide and volatile organic compounds (VOCs)). In turn, high nitrogen (dio)oxide concentrations may favour nitric acid formation as a result of the daytime gas phase recombination reaction of the hydroxyl radical (∙OH) with nitrogen dioxide, and therefore play a key role in secondary inorganic aerosol generation (nitrate aerosols; Masiol et al., 2014). This explains why, when nitrogen dioxide levels are high, levels of other pollutants such as carbon monoxide, volatile organic compounds, and particulate matter will also be high.
Formaldehyde (HCHO) is a volatile organic compound for which satellite observations are available. Sources range from forest fires to furniture, and fuel combustion to personal care products. Health effects from formaldehyde are similar to the ones listed above for particulate matter, nitrogen dioxide, and ozone.
Figure 1.3: Overview of atmospheric physicochemical processes. The red boxes highlight the complexities, nonlinearities, and uncertainties. Primary emissions are denoted by red arrows and secondary reactions are denoted by black arrows. SOA = Secondary Organic Aerosol; hv = solar light. (Molina, L.T., 2021).
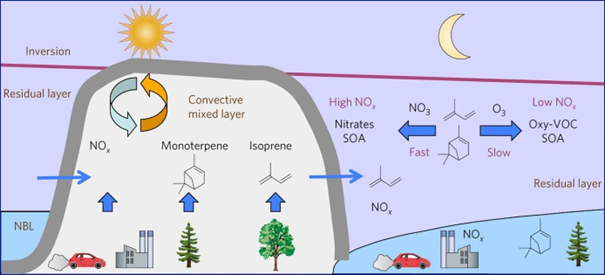
Accumulation of pollutants in urban areas can be exacerbated under certain atmospheric conditions. In winter, a cold spell accompanied by a thermal inversion can favour poor air quality (Figure 1.4). Other contributing factors include frequent and prolonged calm wind periods, high atmospheric stability, persistent thermal inversions, and lower planetary boundary layer heights (during the night). Additionally, there are increased domestic emissions in the coldest months.
Figure 1.4: (Biogenic) volatile organic compounds ((B)VOCs) oxidation by ozone (O3) and nitrate (NO3), emission sources for nitrogen (di)oxide (NOx) and BVOC, and diurnal variation in boundary-layer structure. Although the vertical axis is not to scale, the residual layer constitutes most of the air mass beneath the inversion. (NBL = nocturnal boundary layer; Edwards, P., Aikin, K., Dube, W. et al., 2017).
Question 2
What is the main reason that nitrogen dioxide is an indicator of air quality?